Hydrogen peroxide
Hydrogen peroxide
| Names | |||
|---|---|---|---|
| IUPAC name Hydrogen peroxide | |||
| Other names Dioxidane Oxidanyl Perhydroxic acid 0-hydroxyol Dihydrogen dioxide Oxygenated water Peroxaan | |||
| Identifiers | |||
CAS Number |
| ||
3D model (JSmol) |
| ||
| ChEBI |
| ||
| ChEMBL |
| ||
| ChemSpider |
| ||
| ECHA InfoCard | 100.028.878 | ||
| EC Number |
| ||
IUPHAR/BPS |
| ||
| KEGG |
| ||
PubChem CID |
| ||
| RTECS number |
| ||
| UNII | |||
| UN number | 2015 (>60% soln.) 2014 (20–60% soln.) 2984 (8–20% soln.) | ||
CompTox Dashboard (EPA) | |||
| Properties | |||
| H2O2 | |||
| Molar mass | 34.0147 g/mol | ||
| Appearance | Very light blue color; colorless in solution | ||
| Odor | slightly sharp | ||
| Density | 1.11 g/cm3 (20 °C, 30% (w/w) solution )[1] 1.450 g/cm3 (20 °C, pure) | ||
| Melting point | −0.43 °C (31.23 °F; 272.72 K) | ||
| Boiling point | 150.2 °C (302.4 °F; 423.3 K) (decomposes) | ||
| Miscible | |||
| Solubility | soluble in ether, alcohol insoluble in petroleum ether | ||
| log P | -0.43[2] | ||
| Vapor pressure | 5 mmHg (30 °C)[3] | ||
| Acidity (pKa) | 11.75 | ||
| −17.7·10−6 cm3/mol | |||
Refractive index (nD) | 1.4061 | ||
| Viscosity | 1.245 cP (20 °C) | ||
| 2.26 D | |||
| Thermochemistry | |||
Heat capacity (C) | 1.267 J/(g·K) (gas) 2.619 J/(g·K) (liquid) | ||
Std enthalpy of formation (ΔfH⦵298) | −187.80 kJ/mol | ||
| Pharmacology | |||
| A01AB02 (WHO) D08AX01 (WHO), D11AX25 (WHO), S02AA06 (WHO) | |||
| Hazards | |||
| Safety data sheet | ICSC 0164 (>60% soln.) | ||
| GHS pictograms |    | ||
| GHS Signal word | Danger | ||
| H271, H302, H314, H332, H335, H412 | |||
| P280, P305+351+338, P310 | |||
| NFPA 704 (fire diamond) | |||
| Flash point | Non-flammable | ||
| Lethal dose or concentration (LD, LC): | |||
LD50 (median dose) | 1518 mg/kg[citation needed] 2000 mg/kg (oral, mouse)[4] | ||
LC50 (median concentration) | 1418 ppm (rat, 4 hr)[4] | ||
LCLo (lowest published) | 227 ppm (mouse)[4] | ||
| NIOSH (US health exposure limits): | |||
PEL (Permissible) | TWA 1 ppm (1.4 mg/m3)[3] | ||
REL (Recommended) | TWA 1 ppm (1.4 mg/m3)[3] | ||
IDLH (Immediate danger) | 75 ppm[3] | ||
| Related compounds | |||
Related compounds | Water Ozone Hydrazine Hydrogen disulfide Dioxygen difluoride | ||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |||
| Infobox references | |||
Hydrogen peroxide is a chemical compound with the formula H
2O
2. In its pure form, it is a very pale blue[5] liquid, slightly more viscous than water. Hydrogen peroxide is the simplest peroxide (a compound with an oxygen–oxygen single bond). It is used as an oxidizer, bleaching agent, and antiseptic. Concentrated hydrogen peroxide, or "high-test peroxide", is a reactive oxygen species and has been used as a propellant in rocketry.[6] Its chemistry is dominated by the nature of its unstable peroxide bond.
Hydrogen peroxide is unstable and slowly decomposes in the presence of light. Because of its instability, hydrogen peroxide is typically stored with a stabilizer in a weakly acidic solution in a dark colored bottle. Hydrogen peroxide is found in biological systems including the human body. Enzymes that use or decompose hydrogen peroxide are classified as peroxidases.
Properties[edit]
The boiling point of H
2O
2 has been extrapolated as being 150.2 °C (302.4 °F), approximately 50 °C (90 °F) higher than water. In practice, hydrogen peroxide will undergo potentially explosive thermal decomposition if heated to this temperature. It may be safely distilled at lower temperatures under reduced pressure.[7]
Structure[edit]
Hydrogen peroxide (H
2O
2) is a nonplanar molecule with (twisted) C2 symmetry; this was first shown by Paul-Antoine Giguère in 1950 using infrared spectroscopy.[8][9] Although the O−O bond is a single bond, the molecule has a relatively high rotational barrier of 2460 cm−1 (29.45 kJ/mol);[10] for comparison, the rotational barrier for ethane is 1040 cm−1 (12.5 kJ/mol). The increased barrier is ascribed to repulsion between the lone pairs of the adjacent oxygen atoms.
The approximately 100° dihedral angle between the two O–H bonds makes the molecule chiral. It is the smallest and simplest molecule to exhibit enantiomerism. It has been proposed that the enantiospecific interactions of one rather than the other may have led to amplification of one enantiomeric form of ribonucleic acids and therefore an origin of homochirality in an RNA world.[11]
The molecular structures of gaseous and crystalline H
2O
2 are significantly different. This difference is attributed to the effects of hydrogen bonding, which is absent in the gaseous state.[12] Crystals of H
2O
2 are tetragonal with the space group D4
4P4121.[13]
Aqueous solutions[edit]
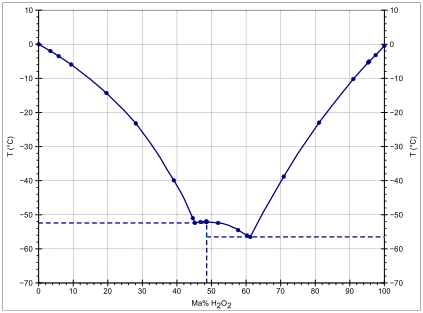
In aqueous solutions, hydrogen peroxide differs from the pure substance due to the effects of hydrogen bonding between water and hydrogen peroxide molecules. Hydrogen peroxide and water form a eutectic mixture, exhibiting freezing-point depression down as low as –56 °C; pure water has a freezing point of 0 °C and pure hydrogen peroxide of −0.43 °C. The boiling point of the same mixtures is also depressed in relation with the mean of both boiling points (125.1 °C). It occurs at 114 °C. This boiling point is 14 °C greater than that of pure water and 36.2 °C less than that of pure hydrogen peroxide.[14]
| H2O2 (w/w) | Density (g/cm3) | Temp. (°C) |
|---|---|---|
| 3% | 1.0095 | 15 |
| 27% | 1.10 | 20 |
| 35% | 1.13 | 20 |
| 50% | 1.20 | 20 |
| 70% | 1.29 | 20 |
| 75% | 1.33 | 20 |
| 96% | 1.42 | 20 |
| 98% | 1.43 | 20 |
| 100% | 1.45 | 20 |
Comparison with analogues[edit]
Hydrogen peroxide has several structural analogues with Hm−X−X−Hn bonding arrangements (water also shown for comparison). It has the highest (theoretical) boiling point of this series (X = O, N, S). Its melting point is also fairly high, being comparable to that of hydrazine and water, with only hydroxylamine crystallising significantly more readily, indicative of particularly strong hydrogen bonding. Diphosphane and hydrogen disulfide exhibit only weak hydrogen bonding and have little chemical similarity to hydrogen peroxide. All of these analogues are thermodynamically unstable. Structurally, the analogues all adopt similar skewed structures, due to repulsion between adjacent lone pairs.
| Name | Formula | Molar mass (g/mol) | Melting point (°C) | Boiling point (°C) |
|---|---|---|---|---|
| Hydrogen peroxide | HOOH | 34.01 | −0.43 | 150.2* |
| Water | HOH | 18.02 | 0.00 | 99.98 |
| Hydrogen disulfide | HSSH | 66.15 | −89.6 | 70.7 |
| Hydrazine | H2NNH2 | 32.05 | 2 | 114 |
| Hydroxylamine | NH2OH | 33.03 | 33 | 58* |
| Diphosphane | H2PPH2 | 65.98 | −99 | 63.5* |
Discovery[edit]
Alexander von Humboldt reported one of the first synthetic peroxides, barium peroxide, in 1799 as a by-product of his attempts to decompose air.
Nineteen years later Louis Jacques Thénard recognized that this compound could be used for the preparation of a previously unknown compound, which he described as eau oxygénée ("oxygenated water") – subsequently known as hydrogen peroxide.[15][16][17] Today, the term "oxygenated water" may appear on retail packaging referring to mixtures containing either water and hydrogen peroxide or water and dissolved oxygen. This could cause personal injury if the difference is not properly understood by the user.[18]
An improved version of Thénard's process used hydrochloric acid, followed by addition of sulfuric acid to precipitate the barium sulfate byproduct. This process was used from the end of the 19th century until the middle of the 20th century.[19]
Thénard and Joseph Louis Gay-Lussac synthesized sodium peroxide in 1811. The bleaching effect of peroxides and their salts on natural dyes became known around that time, but early attempts of industrial production of peroxides failed. The first plant producing hydrogen peroxide was built in 1873 in Berlin. The discovery of the synthesis of hydrogen peroxide by electrolysis with sulfuric acid introduced the more efficient electrochemical method. It was first commercialized in 1908 in Weißenstein, Carinthia, Austria. The anthraquinone process, which is still used, was developed during the 1930s by the German chemical manufacturer IG Farben in Ludwigshafen. The increased demand and improvements in the synthesis methods resulted in the rise of the annual production of hydrogen peroxide from 35,000 tonnes in 1950, to over 100,000 tonnes in 1960, to 300,000 tonnes by 1970; by 1998 it reached 2.7 million tonnes.[20]
Pure hydrogen peroxide was long believed to be unstable, as early attempts to separate it from the water, which is present during synthesis, all failed. This instability was due to traces of impurities (transition-metal salts), which catalyze the decomposition of the hydrogen peroxide. Pure hydrogen peroxide was first obtained in 1894—almost 80 years after its discovery—by Richard Wolffenstein, who produced it by vacuum distillation.[21]
Determination of the molecular structure of hydrogen peroxide proved to be very difficult. In 1892, the Italian physical chemist Giacomo Carrara (1864–1925) determined its molecular mass by freezing-point depression, which confirmed that its molecular formula is H2O2.[22] At least half a dozen hypothetical molecular structures seemed to be consistent with the available evidence.[23] In 1934, the English mathematical physicist William Penney and the Scottish physicist Gordon Sutherland proposed a molecular structure for hydrogen peroxide that was very similar to the presently accepted one.[24][25]
Previously, hydrogen peroxide was prepared industrially by hydrolysis of ammonium persulfate, which was itself obtained by the electrolysis of a solution of ammonium bisulfate (NH
4HSO
4) in sulfuric acid:[26]
Production[edit]
Today, hydrogen peroxide is manufactured almost exclusively by the anthraquinone process, which was formalized in 1936 and patented in 1939. It begins with the reduction of an anthraquinone (such as 2-ethylanthraquinone or the 2-amyl derivative) to the corresponding anthrahydroquinone, typically by hydrogenation on a palladium catalyst. In the presence of oxygen, the anthrahydroquinone then undergoes autoxidation: the labile hydrogen atoms of the hydroxy groups transfer to the oxygen molecule, to give hydrogen peroxide and regenerating the anthraquinone. Most commercial processes achieve oxidation by bubbling compressed air through a solution of the anthrahydroquinone, with the hydrogen peroxide then extracted from the solution and the anthraquinone recycled back for successive cycles of hydrogenation and oxidation.[27][28]
The net reaction for the anthraquinone-catalyzed process is :[27]
- H
2 + O
2 → H
2O
2
The economics of the process depend heavily on effective recycling of the extraction solvents, the hydrogenation catalyst and the expensive quinone.
Other sources[edit]
Small, but detectable, amounts of hydrogen peroxide can be formed by several methods. Small amounts are formed by electrolysis of dilute acid around the cathode where hydrogen evolves if oxygen is bubbled around it. It is also produced by exposing water to ultraviolet rays from a mercury lamp, or an electric arc while confining it in a UV transparent vessel (e.g. quartz). It is detectable in ice water after burning a hydrogen gas stream aimed towards it and is also detectable on floating ice. Rapidly cooling humid air blown through an approximately 2,000 °C spark gap results in detectable amounts.[29]
A commercially viable process to produce hydrogen peroxide directly from the environment has been of interest for many years. Efficient direct synthesis is difficult to achieve, as the reaction of hydrogen with oxygen thermodynamically favours production of water. Systems for direct synthesis have been developed, most of which employ finely dispersed metal catalysts similar to those used for hydrogenation of organic substrates.[30][31] None of these has yet reached a point where they can be used for industrial-scale synthesis.





![{\displaystyle {\ce {2NH4HSO4 ->[{electrolysis}] (NH4)2S2O8 + H2}}}](https://wikimedia.org/api/rest_v1/media/math/render/svg/db83ccf805527a7d814cc0443fd02e172764a05b)
![{\displaystyle {\ce {(NH4)2S2O8 + 2H2O ->[hydrolysis] 2(NH4)HSO4 + H2O2}}}](https://wikimedia.org/api/rest_v1/media/math/render/svg/4ce4047f30e3c22e3987663c1f4a6f4b12e125ee)

Outstanding
ReplyDelete